We use cookies to improve your experience. By accepting you agree to our cookie policy

A research study (Loggia et al., 2015) has been latched onto by the media and heralded using hyperbolic headlines such as “A New Kind of Brain Scan Can See Your Pain, Literally’
‘Literally can see your pain’? Sounds pretty amazing, right? Unfortunately, and as usual, they’ve gotten a little ahead of themselves, and as ground-breaking as the research is, it’s not quite the magical Pain-o-Vision 3000 suggested by the headlines. So let’s take a look at the paper in question and find out what’s really going on…
Glia, such as microglia and astrocytes, are found throughout the brain and spinal cord. They were originally thought to act as nothing more than a kind of glue – their name actually comes from the Greek for glue (γλοία) – that holds neurons in place and does little more.
Fast forward to today, and we know that glia are intimately involved in the workings of the central nervous systems, reacting to trauma and acting to protect the delicate neurons.
Microglia and astrocytes have long been known to activate in response to acute stress – this response involves their multiplication (‘proliferation’), shape changes from fairly relaxed star-like structures to a more compact “ready for action” cells, and the production of cytokines and other inflammatory mediators.
These changes occur to limit tissue damage and restore homeostasis (the natural balance of activity within the body), but sometimes the trigger doesn’t resolve, and these activated microglia can cause problems.
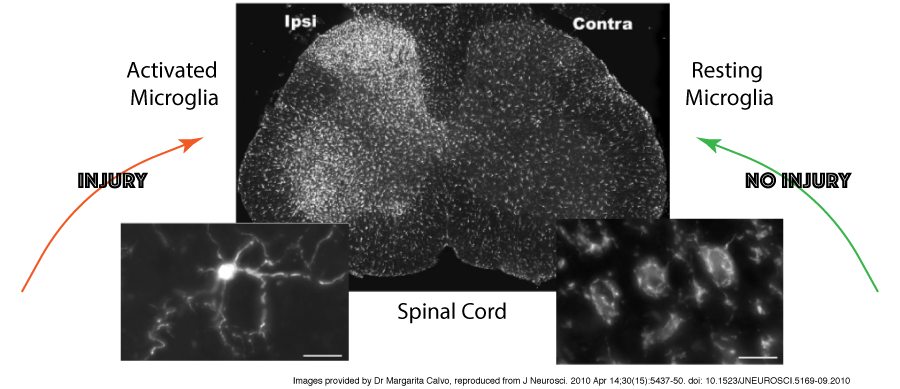
For years, animal models have shown the presence of activated microglia in association with chronic pain, and have even demonstrated that injection of these active cells into a healthy animal can increase their sensitivity to pain, but it has been very difficult to show the same happens in humans.
Post-mortem studies have shown increases of activation and inflammation in patients with CRPS, HIV-associated pain, fibromyalgia, and lower back pain (LBP) – this is promising but also difficult to extrapolate from as the body undergoes many changes around the time of death.
Being able to detect these changes in microglial activation states in a living person is exactly what this paper set out to do. So how did they do it?
The paper was published at the start of the year and comes from a group based at Massachusetts General Hospital. They used a combination of MRI (magnetic resonance imaging – used to look at structural aspects of the body) and PET (positron emission tomography).
PET works by using a ligand (i.e. a molecule that binds to specific receptors) to detect distribution and concentration of specific receptors in the brain – in this case, they looked at translocator protein (TPSO), which is found in the outer membrane of mitochondria and is highly expressed glia such as microglia.
They looked at lower back pain and recruited 19 patients who had been suffering from LBP for 2 or more years, alongside 25 healthy control patients with no history of chronic pain. There were a number of exclusion criteria:
They also looked at the genetic make-up of the study participants, as there is a known polymorphism of TPSO at Ala147Thr (i.e. at amino acid number 147 in the protein chain, sometimes there is an alanine, and sometimes a threonine).
The Thr/Thr mutation is associated with low binding, so individuals with this sequence were excluded. After pairing up the remaining participants, so each LBP patient had a healthy control of the same sex, similar age, and same genetic type, there were 9 pairs (7 Ala/Ala and 2 Ala/Thr).
The McGill Pain questionnaire was used to look at pain, and psychological factors were also assessed using the Beck Depression Inventory (BDI). In addition, blood samples were taken to look at the three most common inflammatory mediators – interleukin 6 (IL-6), interleukin 1 beta (IL-1B), and tumour necrosis factor alpha (TNFA).
They used a 3Tesla (3T) MRI scanner – most MRI scanners currently in use are 1.5T, but they can go up to 7T, and the details they can detect increase with the T-number, so 3T is pretty good! Using MRI in combination with PET allows researchers to see both the “what” (i.e. TPSO binding in PET) and the “where” (i.e. structures highlighted by MRI).
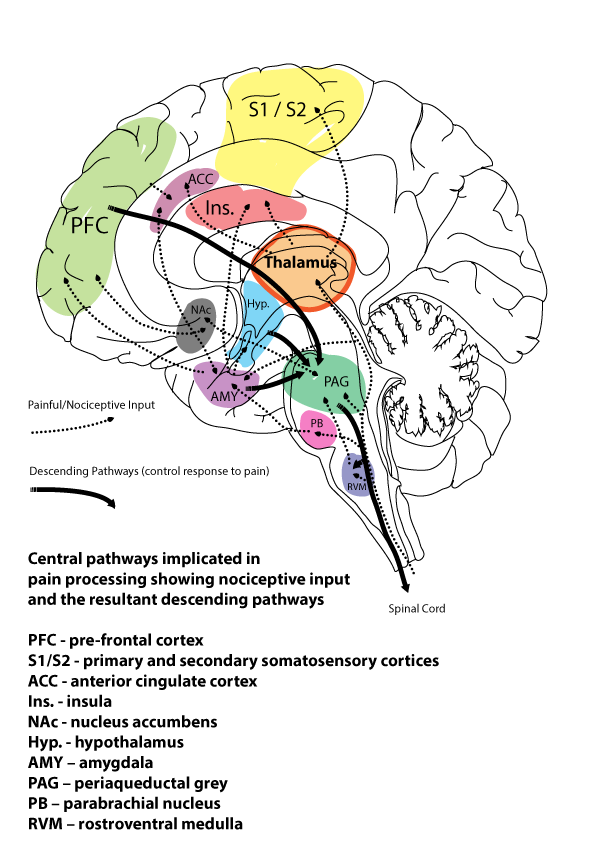
Well, they saw a huge increase in TPSO binding in the thalamus of LBP patients, suggesting activated microglia were present in large numbers in this brain area.
The thalamus is widely recognised as a key brain area involved in processing pain signals and is often considered a “gatekeeper” for pain signals coming from elsewhere in the body.
So how did this correlate with the other measures they looked at – cytokines such as IL-6, and the McGill pain questionnaire showed an inverse relationship (a negative correlation), meaning that low pain scores and decreased levels of IL6 in the blood were associated with stronger TPSO activation in the thalamus.
While this might sound back to front, it is actually a sign that the microglia are responding to an on-going pain situation, and the more strongly they activate, the lower the pain experienced.
However, the situation is slightly different in the healthy group, as they also have low pain scores, but without any increase in thalamic TPSO, reflecting low levels of microglial activation as would be expected for someone who is healthy.
This is the big question really… well, it’s promising, but always worth bearing in mind that this is the first study of its type, and had relatively small group sizes.
Previous studies have hinted at increased microglial activation in CRPS, so it is likely that studies are already being planned to look at the PET/MRI profile in a whole range of other pain conditions, with the aim of determining if all pain conditions show the same thalamic increases, or whether there is a unique signature of activation which is different for each pain condition, and therefore could be used for diagnosis.
In general, this study adds more evidence to the suggestion that activated microglia are involved in the generation of pain, and that treatments that increase TPSO expression even further could have the potential to reduce pain.
At the moment, the main drugs being investigated are minocycline (an antibiotic with neuroprotective effects if given around the time of injury), propentofylline (a xanthine derivative with neuroprotective effects, used to treat Alzheimer’s disease), and low-dose naltrexone (a synthetic opioid antagonist, used at normal dose to treat opioid- and alcohol- dependence, but which has shown some efficacy against fibromyalgia at low doses), although none of these are yet in common use in the clinic.
However, this latest study combining MRI and PET to look at microglial activation offers a possible way to monitor how these drugs work. I predict many more studies into the neuroimmune aspects of chronic pain in the next few years, which can only be of benefit to CRPS sufferers!
Dr Rosie Morland recently completed a PhD in neuroscience and pain studies from Imperial College, and has a special interest in how animal models can help increase understanding of complex pain disorders. Visit ROSIE’S BLOG for more details for more details.
THANK YOU DR R. MORLAND for your excellent article ‘Seeing Pain’ in the Burning Nights CRPS Support series of “Research Studies Explained” and Burning Nights does hope that now CRPS and chronic pain patients have a better understanding about this new type of brain scan that possibly may see your pain.
Have you read a research study concerning CRPS/RSD and chronic pain or a topic that you would like to understand and learn more about? If so please do get in contact with Burning Nights and Dr Morland will take a look and maybe next time your research study topic may be on closer inspection!
Please do share this fantastic article blog by Dr Morland, Research Studies Explained 3, on social media by using the buttons below the blog!
Last Updated: 18/01/2020
We use cookies to improve your experience. By accepting you agree to our cookie policy
 £
£