We use cookies to improve your experience. By accepting you agree to our cookie policy

Chronic pain affects more than one-third of the UK population and is the leading cause of disability. Many patients require daily opioids for pain control, which can result in dependency and misuse.
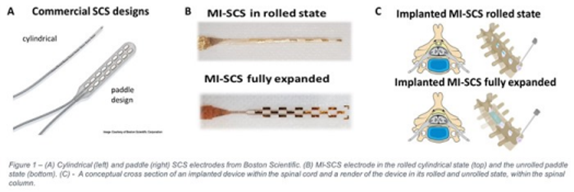
When patients don’t respond to non-invasive medical management, spinal cord stimulation is routinely used. Spinal Cord Stimulators (SCS) are devices implanted into the spine to alter nerve signals in the spinal cord. However, current devices have a 5-10% complication rate and can be expensive and difficult to insert.
There are currently two different types of SCS. The most effective (paddle) type requires major surgery to implant, which is risky and limits the number of suitable patients. Another (cylindrical) type can be implanted through the skin in outpatient settings but is less effective and is often displaced.
We have developed a new SCS device that is as effective at reducing pain as the paddle SCS and as safe and easy to implant as the cylinder SCS. We believe it will make the benefits of effective SCS pain relief available to many more patients. The implant is designed to be accurately positioned inside the spinal column in a simple outpatient procedure under local anaesthetic rather than through major spinal surgery.
The finalised prototype will be ready to undergo testing for regulatory approval at the end of the project, including pre-clinical and clinical trials. We hope that a device will be in mass manufacture and available for use within five years.
Aims of the MI-SCS Patient and Public Involvement (PPI) Advisory Group
PPI is important to health research because representatives provide researchers with insights into what it is like to live with a health condition. By involving a PPI panel in this study, we will ensure its aims and objectives are focused on issues that matter to patients, carers and the public. It will also help with the dissemination of the research beyond the scientific community.
Overview of Role
We are seeking to recruit an Advisory Group of 6 to 8 members of the public to represent people suffering from chronic pain. The group will partner with the MI-SCS research team to promote and advance the use of the device going forward.
Experience / Responsibilities
Members should be people with various lived experiences of chronic pain and life experiences. They should be interested in clinical research but do not need to have participated in research previously as training and support will be provided.
We ask members to make a reasonable effort to attend and prepare for meetings, and to take an active role in group discussions. If unable to attend, PPIE facilitator should be informed prior to the meeting.
Time commitment / Methods of Engagement
Advisory Group members will be invited to quarterly meetings (held virtually), attended by the PPI Lead and Public Co applicant. Meetings will begin with a progress update. Contributors will then lead the discussion, but directions will include 1) Needs/priorities of people living with chronic pain, 2) Device commercialisation, 3) Publication/dissemination plan.
We also aim to hold a bespoke event with local community groups, such as the Chronic Pain Support Group in Suffolk.
Expenses
Members will be reimbursed for reasonable out-of-pocket expenses as a result of helping with this research e.g. travel and parking. Please retain all receipts.
Confidentiality
The PPIE facilitators will always keep members’ personal information secure and confidential and email addresses will be hidden when circulating group emails.
To Apply
Thank you for taking the time to consider this role. If you would like to apply, please contact Lucy de Wesselow on ld575@cam.ac.uk , explaining why you wish to be involved and what they would like to gain from the project.
Closing Date
This study closes on 31st March 2023.
We use cookies to improve your experience. By accepting you agree to our cookie policy
 £
£